Fleqsibility in motion
FLEQSUVY® is an FDA-approved ready-to-use* oral liquid baclofen that may meet your needs for relief of spasticity from multiple sclerosis, spinal cord injuries and other spinal cord diseases.
*Shake well before taking

FLEQSUVY® (baclofen oral suspension) is indicated for:
- The treatment of spasticity resulting from multiple sclerosis, particularly for the relief of flexor spasms and concomitant pain, clonus, and muscular rigidity.
- FLEQSUVY may also be of some value in patients with spinal cord injuries and other spinal cord diseases.
Limitations of Use: FLEQSUVY is not indicated in the treatment of skeletal muscle spasm resulting from rheumatic disorders.
A calibrated measuring device is recommended to measure and deliver the prescribed dose accurately. A household teaspoon or tablespoon is not an adequate measuring device. Discard unused portion 2 months after first opening.

Azurity specializes in providing innovative products that address the specific needs of underserved patient segments, including a broad portfolio of alternative dosage formulations of established FDA-approved products.
Learn More About AzurityUnderstanding spasticity
Spasticity in Multiple Sclerosis (MS)
Spasticity is a tightness or stiffness of the muscles that help people to stand and balance in an upright position. These muscles may be in the legs, groin, and buttocks. Some people may have spasticity in their backs.1
Spasticity in MS happens when there is damage to the myelin—the protective coating covering the nerves of the brain and spinal cord. These nerves control movement. Mild spasticity may not be painful. But spasticity can become painful when it is more severe. Spasticity can also make it hard to perform daily activities because of discomfort or difficulty moving.1
Spasticity in Spinal Cord Injury (SCI)
Spasticity in SCI happens when the flow of signals between the brain and spinal cord is broken. In a normal response, information travels from the spinal cord to the brain. The brain receives the signal and sends commands back through the spinal cord to tell the body what to do. A reaction like jerking away from something hot is a reflex, and it happens automatically. In SCI, the signal can’t reach the brain. It goes back to the spinal cord and the reflex is a muscle spasm.2
Symptoms are similar to the symptoms for MS.
They can include2:
- Sudden bending or straightening of limbs or jerking of muscles
- Muscle spasms
- Stiffness or tight muscles at rest or during activity that makes it hard to relax or to control movements
How common is spasticity?
If you have spasticity, you are not alone.
Percentage of people in the US with spasticity
Multiple Sclerosis3
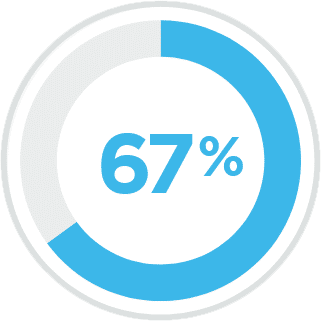
Spinal Cord Injury3
Understanding dysphagia
If you have spasticity, you may also have dysphagia—any difficulty in swallowing.4 This may affect swallowing food and also medicine like pills or capsules. Dysphagia may happen in different areas—when you first feel something in your mouth, move it around in your mouth or actually try to swallow.5
Percentage of people in the US with dysphagia
Multiple Sclerosis4
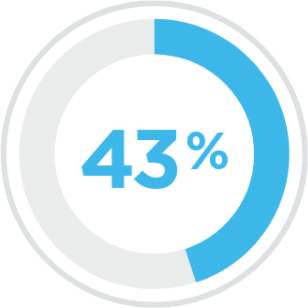
Spinal Cord Injury5


Did you know?
Baclofen has been prescribed as a treatment for spasticity for over 40 years and is a commonly used medicine for treating spasticity in MS.1,6
About FLEQSUVY®—a liquid form of baclofen
FLEQSUVY® provides:
Grape flavor
May help with the taste
Flexible dosing
Get the right dose your doctor tells you to take, every time†
Ease in taking your oral liquid
Oral liquid may help you keep taking your medication
Consistent potency
From the first drop to the last
Easy to store
No need to refrigerate or keep cold. Throw away any unused medicine 2 months after opening
†A calibrated measuring device is recommended to measure and deliver the prescribed dose accurately. A household teaspoon or tablespoon is not an adequate measuring device. Discard unused portion 2 months after first opening.
A different option for taking your medicine
If you need to take medicine for spasticity—and you also have difficulty swallowing pills—a liquid form of baclofen may make it easier for you to take your medicine.
FLEQSUVY® is a ready-to-use‡
oral liquid suspension, which means no need for:
 crushing
crushing  compounding
compounding ‡Shake well before taking.
Talk to your doctor to see if FLEQSUVY® may be right for you.
Taking FLEQSUVY®
FLEQSUVY® is a liquid form of baclofen. It contains 25 mg per 5 mL—or 5 mg/mL—of baclofen as an orange- to yellow-colored, grape-flavored liquid. Because FLEQSUVY® is a suspension, you need to shake the bottle thoroughly to make sure all of the baclofen particles are equally spread out throughout the liquid. This will ensure that you are taking the right amount of medication.
- Your doctor will decide how much FLEQSUVY® you need to take. FLEQSUVY® is a medicine where you start at a lower amount and your dose is slowly increased until you take the amount of medicine your doctor tells you to take
- You can take FLEQSUVY® with or without food
- FLEQSUVY® does not have to be refrigerated or kept cold. Keep FLEQSUVY® at room temperature
- You must throw away the bottle 2 months after opening it—even if there is medicine left inside
Tips to help you manage your spasticity
- Let your doctor know how you are feeling about your spasticity and about your medicine
- Understanding your condition is important. Work with your doctor to answer any questions or concerns you may have
- If you don’t understand what the doctor is telling you, say so. You may need more help to understand
- It is important to take your medicine as your doctor tells you to
Pharmacists can provide useful information and support. They can:
- Provide a measuring device that you can use to measure your medicine as your doctor tells you to
- Explain what your medicine is for and answer questions you have on how to take it
Resources
Below are organizations that may be able to support you and your family.

National Foundation of Swallowing Disorders
The National Foundation of Swallowing Disorders is a nonprofit, patient-advocacy organization for those impacted by dysphagia. The organization is committed to providing patients hope and helping to improve quality of life for those suffering from all types of swallowing disorders by enhancing direct patient support, education, and research and by raising public, professional, and governmental awareness.
Learn More About National Foundation of Swallowing Disorders
Multiple Sclerosis Association of America
The Multiple Sclerosis Association of America (MSAA) is a leading resource for the entire MS community, improving lives today through vital services and support. MSAA is a national nonprofit organization founded in 1970 and provides ongoing support and direct services to individuals with MS, their families, and their care partners.
Learn More About Multiple Sclerosis Association of America
Can Do Multiple Sclerosis
Can Do Multiple Sclerosis is a national organization that delivers health and wellness education programs on exercise, nutrition, symptom management, and motivation to help families living with multiple sclerosis thrive.
Learn More About Can Do Multiple Sclerosis
MS Focus
The Multiple Sclerosis Foundation, known as MS Focus, provides free services that address the critical needs of people with multiple sclerosis and their families to help them maintain the best quality of life.
Learn More About MS FocusImportant Safety Information for FLEQSUVY® (baclofen oral suspension)
INDICATIONS AND USAGE:
- FLEQSUVY is indicated for the treatment of spasticity resulting from multiple sclerosis, particularly for the relief of flexor spasms and concomitant pain, clonus, and muscular rigidity.
- FLEQSUVY may also be of some value in patients with spinal cord injuries and other spinal cord diseases.
Limitations of Use: FLEQSUVY is not indicated in the treatment of skeletal muscle spasm resulting from rheumatic disorders.
FLEQSUVY is a concentrated formulation. Verify the strength and the dose of the product prior to dispensing and administering. Use an oral dosing syringe and not a household teaspoon to correctly measure the prescribed amount of medication. Oral dosing syringes may be obtained from your pharmacy. Shake well before using.
Important Safety Information
Do not take FLEQSUVY if you have a hypersensitivity to baclofen.
WARNINGS AND PRECAUTIONS:
Do not discontinue FLEQSUVY without consulting your healthcare provider. Sudden withdrawal of FLEQSUVY can result in serious complications that include hallucinations, seizures, high fever, confusion, muscle stiffness, multiple organ-system failure, and death.
Early symptoms of FLEQSUVY withdrawal may include increased spasticity, itching, and tingling of extremities. Call your doctor right away if you have any signs of FLEQSUVY withdrawal.
Do not drive or operate heavy machinery after taking your dose of FLEQSUVY until you know how it affects you. FLEQSUVY may cause drowsiness. Do not operate other dangerous machinery or engage in activities made hazardous by decreased alertness until you know how FLEQSUVY affects you. Do not increase your dose until advised to do so by your healthcare provider and you know how the drug affects you.
Drowsiness associated with FLEQSUVY can be worsened by alcohol and other CNS depressants.
Ovarian cysts have been found by palpation in about 4% of the multiple sclerosis patients who were treated with oral baclofen for up to one year. In most cases, these cysts disappeared spontaneously while patients continued to receive the drug.
Before taking FLEQSUVY, tell your healthcare provider if you:
- have a hypersensitivity to baclofen or any component of FLEQSUVY
- have or have had a stroke. Baclofen has not significantly benefited patients with stroke. These patients have also shown poor tolerability to the drug
- have or have had seizures. FLEQSUVY can cause a worsening of this condition
- have or have had history of psychotic disorders, schizophrenia, or confusional states. FLEQSUVY can cause a worsening of these conditions
- have or have had history of autonomic dysreflexia. FLEQSUVY can cause a worsening of this condition
- are pregnant, plan to become pregnant, or plan to breastfeed. Withdrawal symptoms in neonates whose mothers were treated with oral baclofen throughout pregnancy have been reported. You and your healthcare provider will decide if you should take FLEQSUVY while you are pregnant
- have or have had difficulty with balance, or sustaining upright posture
- have or have had kidney problems or are on hemodialysis
- have any other medical conditions
Read all medicine labels carefully and tell your healthcare provider about all medicines you take, including prescription and non-prescription medicines.
Store FLEQSUVY at room temperature and discard unused portion 2 months after first opening.
The most common side effects of FLEQSUVY include drowsiness, dizziness, and weakness. Tell your healthcare provider about any side effect that bothers you or does not go away. These are not all the possible side effects of FLEQSUVY.
The Important Safety Information does not include all the information needed to use FLEQSUVY safely and effectively. Please talk to your healthcare professional and see complete Prescribing Information for FLEQSUVY.
To report SUSPECTED ADVERSE REACTIONS, contact Azurity Pharmaceuticals, Inc. at 1-800-461-7449, or FDA at 1-800-FDA-1088 or www.fda.gov/MedWatch.
PP-FLE-US-0090
References
- 1. Spasticity (stiffness). Multiple Sclerosis Association of America website. Visit https://mymsaa.org/ms-information/symptoms/spasticity/. Accessed December 18, 2021.
- 2. Spasticity and Spinal Cord Injury. Model Systems Knowledge Translation Center website. Visit https://msktc.org/sci/factsheets/Spasticity#:~:text=Authorship-. Accessed February 22, 2022.
- 3. McGuire J. Epidemiology of spasticity in the adult and child. In Brashear A, Elovic E, eds. Spasticity Diagnosis and Management. 2nd ed. New York, NY: Demos Medical; 2016:5-15.
- 4. Ansari NN, Tarameshlu M, Ghelichi L. Dysphagia in multiple sclerosis patients: diagnostic and evaluation strategies. Degener Neurol Neuromuscul Dis. 2020;10:15-29. doi:10.2147/DNND.S198659
- 5. Dysphagia. Spinal Cord Injury Research Evidence Project (SCIRE) website. Visit https://scireproject.com/evidence/rehabilitation-evidence/nutrition-issues-following-spinal-cord-injury/dietary-int-0/#:~:text=The prevalence of dysphagia.2. Accessed January 24, 2022.
- 6. FLEQSUVY® [package insert]. Woburn, MA: Azurity Pharmaceuticals, Inc; 2023.